What is a MotherToBaby Pregnancy Study? Who is MotherToBaby?
A MotherToBaby Pregnancy Study is a research study that collects information from a woman during her pregnancy along with information about her baby’s health after the pregnancy is over. MotherToBaby Pregnancy Studies are conducted by the non-profit Organization of Teratology Information Specialists (OTIS) and are coordinated by the University of California, San Diego.
Will I be asked to take any additional medications? Or to stop taking a medication?
No. Our studies are strictly observational, which means we will never ask you to take any new medicines, experimental drugs or to change any part of your healthcare routine. If you enroll, you’ll simply be followed by our team through the remainder of your pregnancy, and your baby will be followed for a period of time after birth.
Why are pregnancy studies important?
Pregnant women are typically excluded from clinical trials that occur when a drug is being developed. This means that once a medication is approved by the US Food & Drug Administration (FDA), it starts being prescribed to patients with little to no information on the safety of the drug if used during pregnancy. Yet 9 out of 10 pregnant women in the U.S. take medication. Our studies provide critical information on the safety of medication use by observing pregnant women and their babies. Our findings are used by healthcare providers and may be listed on drug labels that come with prescription medications.
How does a MotherToBaby Pregnancy Study work?
After you let us know that you are interested, the first thing that will happen is that we will contact you to get some information and to see if you are eligible to participate. The 3 groups of women we’re looking for are:
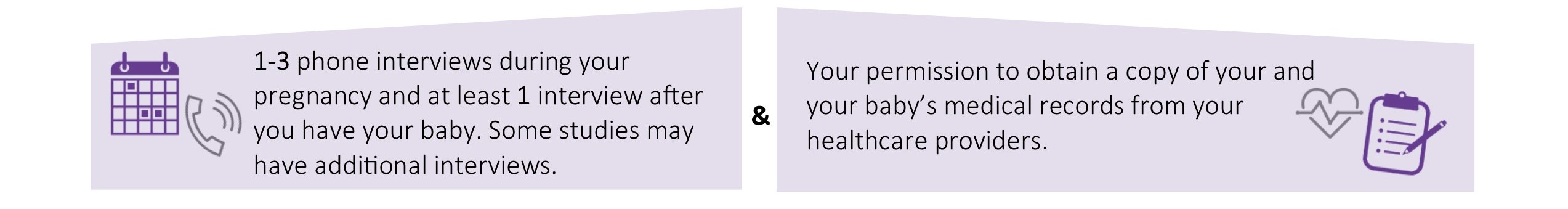
If you’re eligible to join one of these groups, we’ll tell you exactly what is involved in the study so you can decide if you want to participate. All of our studies include:
Depending on the study you join, you may also be eligible for a free in-home examination of your baby by one of our pediatric specialists and/or free neurodevelopmental follow-up until your child is 5 years old.
Visit our website (https://mothertobaby.org/studies/) for a list of our ongoing studies and to watch a video of a past participant describing her experience being in a MotherToBaby study.
Will the information I share with MotherToBaby be kept private?
All the information we request will be kept confidential. Your privacy is important to us. We comply with strict regulations and laws set forth by state and federal agencies as well as the Institutional Review Board (IRB) at the University of California, San Diego, who oversees and monitors our research. Since we ask for your permission to obtain medical records, we will also give you detailed information on how this data will be used and how we will protect your privacy.
What types of questions will you ask me?
We’ll ask you questions about your health history, your pregnancy and the birth of your child. You’ll be asked about exposures to medications, illnesses, pregnancy related tests, previous pregnancies, medical condition symptom control (if participating in group 1 or 2), and questions about demographics. After your baby is born, we may also ask you questions about your breastfeeding practices (should you choose to nurse your baby) as well as questions about your child’s development.
What are the benefits of participating?
Will I find out the results of the study?
Yes! We send all participants and their health care providers the results of the study. This information might prove useful to you in a future pregnancy, or be used by your provider when treating other patients.
What if I agree to participate and later change my mind?
Because your participation is voluntary, you can withdraw from the study at any time.
I have more questions. Who can I contact?
Contact MotherToBaby Pregnancy Studies by:
- Phone (toll-free): 877-311-8972 (Monday-Thursday 7am-7pm Pacific; Friday 7am-6pm Pacific)
- Email: mothertobaby@ucsd.edu
- Visit: https://mothertobaby.org/join-study/
OTIS/MotherToBaby encourages inclusive and person-centered language. While our name still contains a reference to mothers, we are updating our resources with more inclusive terms. Use of the term mother or maternal refers to a person who is pregnant. Use of the term father or paternal refers to a person who contributes sperm.